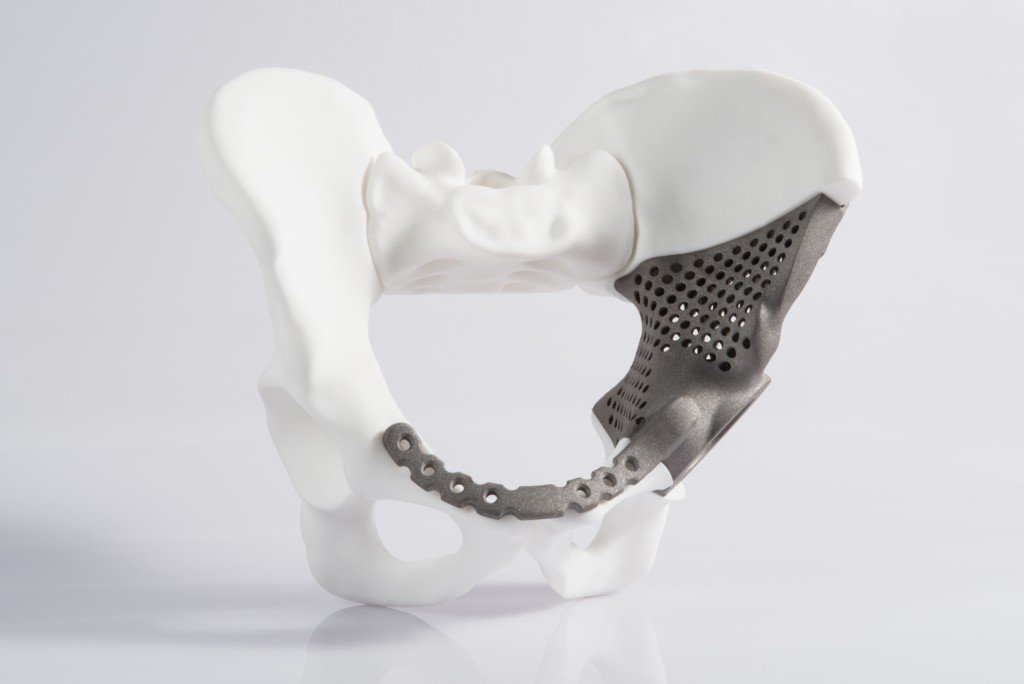
The U.S. medical device industry is considered by many to be one of the country’s most innovative business sectors. Not surprisingly, due to the nature of medical devices and the critical issues they address, it is also one of the most highly regulated.
The Food and Drug Administration’s (FDA) Center for Devices and Radiological Health (CDRH) is the government agency responsible for regulating the production of medical devices, including those manufactured through 3D printing – a process on the leading edge of innovation in the industry.
As more medical applications for 3D printing are discovered and the technology continues to rapidly evolve, the FDA and 3D printing represent a critical intersection of compliance and innovation in the industry.
In this article, we’ll provide an overview of the FDA’s regulations for the 3D printing of medical devices and examine the agency’s unique relationship with the advancement of additive manufacturing technology.
FDA Regulations for Metal 3D Printing
The FDA’s CDRH regulates firms that manufacture, repackage, relabel, and/or import medical devices sold in the United States. According to the agency, some requirements “apply to medical devices before they are marketed (premarket requirements), and others apply to medical devices after they are marketed (postmarket requirements).”
Medical devices are divided into three classifications – Class I, II, and III – which represent the associated risk to the patient and define the regulatory requirements for a general device type, such as premarket notification and approval. Naturally, regulatory control increases from Class I to Class III.
In a great additive manufacturing study published by the Deloitte Center for Industry Insights, the following examples are provided for devices in each category:
- Class I (low risk to the patient) – Nasal oxygen cannulas, manual stethoscopes and hand splints
- Class II (moderate risk to the patient, more invasive and typically implanted into the body) – Tracheal tubes, bone plates, and elbow joint radial prostheses
- Class III (highest risk to the patient) – Aortic valves, constrained metal hip prostheses and coronary stents
Supporting Innovation or Preventing Progress?
As we discussed in a recent article about metal 3D printing in medical, the FDA has struggled to keep pace with both the innovation occurring in 3D printing and the overwhelming number of approval requests for new, 3D-printed medical devices. Fortunately, the agency recognizes that its inability to match the rapid pace of innovation and process requests must not serve as an impediment to progress; especially when that progress can literally mean the difference between life and death for a patient. To address this gap, the agency allows for investigational device exemptions (IDEs).
According to risk management and advisory firm, Willis Towers Watson, the two most common IDE provisions for 3D printed devices are:
- Expanded Access (also known as “Compassionate Use”) – Allows for the use outside of a clinical trial of a medical device product that has not been approved by the FDA. This provision is “typically approved for individual patients with a serious disease and no alternative treatment but may be approved to treat a small group.”
- Emergency Use – This provision is designed for situations “in which there will be a need to use an investigational device in a manner inconsistent with the approved investigational plan or by a physician who is not part of the clinical study.” To qualify, “the condition must be life-threatening or serious, the physician must also have no alternative treatment and the situation has to leave no time to obtain FDA approval.”
Innovating in the Realm of the Unregulated
Some companies are choosing to make strides and innovate in areas that are not regulated by the FDA, but still offer ample opportunities for valuable medical contributions.
The Deloitte Center for Industry Insights cites the story of a five-day-old baby boy born with a life-threatening congenital heart defect in April 2016. Physicians turned to a 3D-printed model of the baby’s heart when CT scans lacked the detail and visibility necessary to confidently prepare for the boy’s life-saving procedure.
The Bottom Line for Metal AM in Healthcare
While FDA regulations present their fair share of challenges, many device companies are utilizing 3D printing technology in truly innovative ways to advance patient care and ensure positive health outcomes for a variety of individuals.
3DEO is Metal Additive Manufacturing
3DEO is proud to partner with such companies in manufacturing their devices through our Intelligent Layering® metal additive manufacturing process, which offers a cost-effective metal 3D printing solution for a variety of products in both small and large batches.
Contact us today to learn how 3DEO is delivering high-quality, low-cost products with immediate turnaround for a wide variety of medical device manufacturers.